Evidence That Shows Agains the Origin of Eucaryotes
- Review
- Published:
The origin and early on evolution of eukaryotes in the calorie-free of phylogenomics
Genome Biology volume xi, Commodity number:209 (2010) Cite this commodity
Abstract
Phylogenomics of eukaryote supergroups suggest a highly complex last common ancestor of eukaryotes and a key role of mitochondrial endosymbiosis in the origin of eukaryotes.
Eukaryotes
The origin of eukaryotes is a huge enigma and a major challenge for evolutionary biology [1–3]. There is a sharp divide in the organizational complication of the cell betwixt eukaryotes, which have complex intracellular compartmentalization, and even the nigh sophisticated prokaryotes (archaea and leaner), which do not [4–6]. A typical eukaryotic jail cell is about 1,000-fold bigger by book than a typical bacterium or archaeon, and functions nether different physical principles: free diffusion has little function in eukaryotic cells, just is crucial in prokaryotes [7, 8]. The compartmentalization of eukaryotic cells is supported past an elaborate endomembrane system and by the actin-tubulin-based cytoskeleton [9, 10]. At that place are no direct counterparts of these organelles in archaea or bacteria. The other authentication of the eukaryotic cell is the presence of mitochondria, which have a fundamental role in energy transformation and perform many boosted roles in eukaryotic cells, such as in signaling and cell death.
The conservation of the major features of cellular organization and the beingness of a large gear up of genes that are conserved across eukaryotes get out no incertitude that all extant eukaryotic forms evolved from a last eukaryote mutual ancestor (LECA; encounter below). All eukaryotes that take been studied in sufficient detail possess either mitochondria or organelles derived from mitochondria [11–thirteen], so it is thought that LECA already possessed mitochondria (see below). Plants and many unicellular eukaryotes also have another type of organelle, plastids.
The organizational complication of the eukaryotic cells is complemented by extremely sophisticated, cross-talking signaling networks [14]. The main signaling systems in eukaryotes are the kinase-phosphatase mechanism that regulates protein function through phosphorylation and dephosphorylation [15–18]; the ubiquitin network that governs poly peptide turnover and localization through reversible protein ubiquitylation [nineteen–21]; regulation of translation by microRNAs [22–24]; and regulation of transcription at the levels of individual genes and chromatin remodeling [24–27]. Eukaryotes all share the main features of cellular architecture and the regulatory circuitry that clearly differentiate them from prokaryotes, although the bequeathed forms of some signature eukaryotic systems are increasingly detected in prokaryotes, equally discussed below. Phylogenomic reconstructions bear witness that the feature eukaryotic complexity arose about 'ready fabricated', without any intermediate grades seen between the prokaryotic and eukaryotic levels of organization [9, 28–30]. Explaining this apparent leap in complexity at the origin of eukaryotes is one of the principal challenges of evolutionary biology.
The key to the origin of eukaryotes will undoubtedly be institute using comparative genomics of eukaryotes, archaea and bacteria. Complete genome sequences from all three domains of cellular life are accumulating exponentially, albeit at markedly different paces. As of March 2010, the NCBI genome database contained over 1,000 bacterial genomes, well-nigh 100 archaeal genomes, and about 100 genomes of eukaryotes [31]. Hither, I hash out some of the main insights that have come up from comparative assay of these genomes, which may help to shed light on the origin and the early stages of evolution of eukaryotes. So far, the comparative genomics era has brought fascinating clues but no decisive breakthrough.
The supergroups of eukaryotes and the root of the eukaryotic evolutionary tree
Although several eukaryotic kingdoms, such as animals, fungi, plants and ciliates, are well defined and seem to be monophyletic beyond reasonable doubt, deciphering the evolutionary relationships between these kingdoms and numerous other groups of unicellular eukaryotes (also chosen protists) turned out to exist daunting. For many years, evolutionary biologists tended to favor the and then chosen crown group phylogeny [2, 32]. The 'crown' of this evolutionary tree included animals (Metazoa) and plants (Viridiplantae), fungi and various assortments of protists, depending on the methods used for tree construction [33, 34]. The residuum of the protists, such as microsporidia, diplomonads and parabasalia, were considered 'early branching eukaryotes'; for some of them, this conclusion was reached considering they appeared to lack mitochondria and were therefore thought to have evolved before the mitochondrial symbiosis. The scenario resulting from the crown group phylogeny was called the archezoan scenario: the archaezoan was divers as a hypothetical ancestral form that lacked mitochondria but possessed the other signature features of the eukaryotic cell. All the same, during the by decade, the early on branching groups accept lost their positions at the root of the eukaryotic tree, ane afterward another [35–37]. The improved taxon sampling as a effect of genome sequencing together with new, more robust methods for phylogenetic assay indicate that the deep placing of these groups seen in early trees was a long-branch artifact acquired by the fast evolution of the respective organisms [37–39]. At the same time, comparative-genomic and ultrastructural studies destroyed the biological underpinning of the near-root positions of the (old) early branching groups of protists past showing that none of them ancestrally lack mitochondria, as they all take genes of apparent mitochondrial origin and mitochondria-related organelles, such every bit hydrogenosomes and mitosomes [xi, 12, thirteen, 40].
There are therefore no grounds to consider any group of eukaryotes primitive, a presymbiotic archezoan. Rather, taking into account the small genomes and high rate of evolution feature of virtually of the protist groups thought to be early on branching, and their parasitic lifestyle, it is becoming increasingly articulate that most or maybe all of them evolved from more than complex ancestral forms by reductive evolution [37, 39]. Reductive evolution refers to the evolutionary modality typical of parasites: they tend to lose genes, organelles and functions when the respective functionalities are taken over past the host. And so the archezoan (crown group) phylogeny seems to take been disproved, and deep phylogeny and the theories of the origin of eukaryotes finer had to start from scratch.
This time phylogenomic approaches were mainly used, that is, phylogenetic analysis of genome-broad sets of conserved genes; this was made possible by the much larger number of genomes that had been sequenced [41, 42]. The key accomplishment at this new phase was the proposal of 'supergroups' of eukaryotes that are suggested to combine highly diverse groups of organisms in a monophyletic grouping [36, 43–45]. Most of the phylogenomic analyses published so far converge on five supergroups (or vi if the Amoebozoa and Opisthokonts do not form a unmarried supergroup, the Unikonts; Figure 1). Although proving monophyly is non-niggling for these groups [46–48], the general structure of the tree, with a few supergroups forming a star-similar phylogeny (Figure 1), is reproduced consistently, and the latest results [49–52] seem to support the monophyly of the five supergroups.

Evolution of the eukaryotes. The relationship between the five eukaryotic supergroups - Excavates, Rhizaria, Unikonts, Chromalveolates and Plantae - are shown equally a star phylogeny with LECA placed in the heart. The 4,134 genes assigned to LECA are those shared by the free-living excavate amoeboflagellate Naegleria gruberi with representatives of at least one other supergroup [67]. The numbers of these putative ancestral genes retained in selected lineages from different supergroups are as well indicated. Branch lengths are arbitrary. Two putative root positions are shown: I, the Unikont-Bikont rooting [56, 57]; Two, rooting at the base of operations of Plantae [60].
The relationship betwixt the supergroups is a formidable problem as the internal branches are extremely brusk, suggesting that the radiation of the supergroups occurred apace (on the evolutionary scale), perhaps resembling an evolutionary 'large bang' [53–55]. Two recent, independent phylogenetic studies [51, 52] each analyzed over 130 conserved proteins from several dozen eukaryotic species and, afterward exploring the effects of removing fast-evolving taxa, arrived at a 3-megagroup construction of the eukaryotic tree. The megagroups consist of Unikonts, Excavates, and the assemblage of Plantae, Chromalveolata and Rhizaria [51, 52].
Furthermore, there take been several attempts to infer the position of the root of the eukaryotic tree (Figure i). The first culling to the crown group tree was proposed by Cavalier-Smith and coworkers [56–58], who used rare genomic changes (RGCs) [59], such as the fusion of two enzyme genes [56, 57] and the domain construction of myosins [58], to place the root between the Unikonts and the rest of eukaryotes (I (red arrow) in Figure one). This separation seems biologically plausible because Unikont cells have a single cilium, whereas all other eukaryotic cells accept 2. Nonetheless, this determination could be suspect because the employ of but a few RGCs makes it difficult to rule out homoplasy (parallel emergence of the same RGC, such every bit gene fusion or fission, in different lineages). Rogozin and coworkers [60] used a unlike RGC approach based on rare replacements of highly conserved amino acid residues requiring two nucleotide substitutions and inferred the virtually probable position of the root to be betwixt Plantae and the residuum of eukaryotes (Ii (greenish pointer) in Figure 1). Again, this seems biologically plausible because the cyanobacterial endosymbiosis that gave rising to plastids occurred on the Plantae lineage.
The controversy about the root position and the lack of consensus regarding the monophyly of at least some of the supergroups, let alone the megagroups, indicate that, despite the emerging clues, the deep phylogeny of eukaryotes currently should be considered unresolved. In a sense, given the probable 'big blindside' of early eukaryote radiation, the branching order of the supergroups, in itself, might be viewed as relatively unimportant [61]. Nevertheless, the biological events that triggered these early radiations are of major involvement, so earnest attempts to resolve the deepest branches of the eukaryotic tree will undoubtedly continue with larger and further improved datasets and methods.
The last common antecedent of eukaryotes
Comparative analysis of representative genomes from dissimilar eukaryotic supergroups enables the reconstruction of the cistron complement of LECA using maximum parsimony (MP) or more sophisticated maximum likelihood (ML) methods [62–64]. Essentially, genes that are represented in various extant representatives of different supergroups, even though lost in some lineages, can be mapped back to LECA. The results of all these reconstructions consistently point to a circuitous LECA, in terms of both the sheer number of ancestral genes and, maybe even more importantly, the ancestral presence of the signature functional systems of the eukaryotic cell (run across below). A MP reconstruction based on phyletic patterns in clusters of orthologous genes of eukaryotes mapped 4,137 genes to LECA (Effigy 1) [63, 65, 66]. Remarkably, an even simpler interpretation, based on the recent analysis of the genome of Naegleria gruberi, the first sequenced genome of a free-living excavate [67], revealed about a nearly identical number of genes, 4,134, that are shared by Naegleria and at least ane other supergroup of eukaryotes, suggesting that these genes are part of the LECA heritage (Effigy ane). Such estimates are highly conservative every bit they do not account for lineage-specific loss of ancestral genes, a major aspect in the evolution of eukaryotes. Indeed, even animals and plants, the eukaryotic kingdoms that seem to be the least prone to factor loss, have still lost about xx% of the putative ancestral genes identified in the unicellular Naegleria (Figure i). Given that the current estimate for the cistron complement of LECA must exist conservative, the genome of LECA is likely to have been every bit complex every bit those of typical extant complimentary-living unicellular eukaryotes [68].
This conclusion is supported by reconstructions from comparative genomics of the bequeathed composition of the cardinal functional systems of the LECA, such as the nuclear pore [28, 69], the spliceosome [29], the RNA interference machinery [70], the proteasome and the ubiquitin signaling arrangement [71], and the endomembrane apparatus [10]. The outcomes of these reconstructions are all straightforward and consistent, even when dissimilar topologies of the phylogenetic tree of eukaryotes were used as the scaffold for the reconstruction: LECA already possessed all these structures in its fully functional state, possibly every bit complex as the counterparts in modernistic eukaryotes.
Reconstruction of other aspects of the genomic composition and architecture of LECA similarly points to a highly complex bequeathed genome. Comparative-genomic analysis of intron positions in orthologous genes within and between supergroups suggests loftier intron densities in the ancestors of the supergroups and in LECA, at least equally dense equally in modern free-living unicellular eukaryotes [72–75]. A systematic analysis of widespread gene duplications in eukaryotes indicates that hundreds of duplications predate LECA, particularly duplications of genes involved in poly peptide turnover [63, 65, 66]. Taken together, these results clearly bespeak that LECA was a typical, fully adult eukaryotic cell. The subsequent evolution of eukaryotes has seemingly shown no consistent trend toward increased complexity, except for lineage-specific embellishments, such equally those seen in animals and plants. At that place was evidently an of import stage of evolution on the 'stem' of eukaryotes, subsequently they first evolved but earlier LECA, which included extensive duplication of numerous essential genes, then that the set of ancestral genes approximately doubled [63, 65, 66].
The archaeal and bacterial roots of eukaryotes
Eukaryotes are hybrid organisms in terms of both their cellular system and their factor complement. All eukaryotes seem to possess mitochondria or related organelles derived from α-proteobacteria, whereas Plantae and many groups of Chromalveolata additionally have blue-green alga-derived plastids [76, 77]. The gene complement of eukaryotes is an uneven mix of genes of apparent archaeal origin, genes of probable bacterial origin, and genes that so far seem eukaryote-specific, without convincing testify of beginnings in either of the ii prokaryote domains (Figure two). Paradoxical as this might appear, although trees based on rRNA genes and concatenated alignments of information-processing proteins, such as polymerases or splicing proteins, both put archaea and eukaryotes together, genome-wide analyses consistently and independently show that at that place are three or more times more genes with bacterial homologs than with archaeal homologs [62, 63, 78, 79] (Effigy two). The archaeal subset is strongly enriched in information processing functions (translation, transcription, replication, splicing), whereas the bacterial subset consists largely of metabolic enzymes [62, 78] (see beneath for more details).
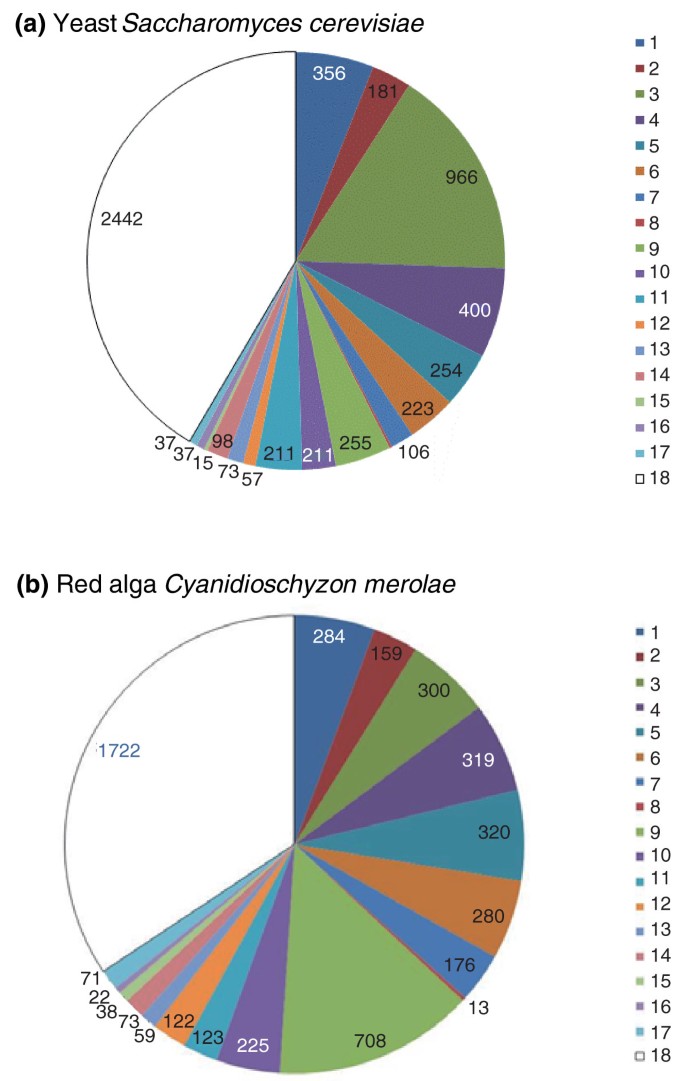
Breakdown of the genes from two eukaryotes by the putative evolutionary affinities. (a) Yeast and (b) red algae. The putative origin of genes was tentatively inferred from the best hits obtained by searching the NCBI non-redundant poly peptide sequence database using the BLASTP program [125], with all poly peptide sequences from the respective organisms used every bit queries. Although sequence similarity searches are often regarded every bit a very rough approximation of the phylogenetic position [126], the previous analysis of the yeast genome showed a loftier level of congruence between the all-time hits and phylogenomic results [78]. Major archaeal and bacterial groups are color-coded and denoted 1 to xviii; the number of proteins with the best hitting to the given groups is indicated. The groups are: i, Euryarchaeota; 2, Crenarchaeota-Thaumarchaeota-Nanoarchaeota; 3, Firmicutes; 4, γ-Proteobacteria; 5, α-Proteobacteria; half dozen, δ- and ε-Proteobacteria; vii, β-Proteobacteria; 8, unclassified Proteobacteria; 9, Blue-green alga; ten, Actinobacteria; 11, Bacteroides-Chlorobi group; 12, Chloroflexi; thirteen, Planctomycetes; 14, Verrucomicrobia-Chlamydiae-Spirochetes; 15, Deinococcus-Thermus group; 16, Aquificacae and Thermotogae; 17, other bacteria; eighteen, no archaeal or bacterial homologs.
At a coarse level, these observations are best compatible with genome fusion scenarios [79, 80] whereby the eukaryotic genome emerged through a fusion betwixt 2 ancestral genomes, an archaeal or archaea-related one, and a bacterial, nearly likely α-proteobacterial, i, given the well-established ancestry of the mitochondrial endosymbiont [81]. However, attempts to pinpoint the specific archaeal and bacterial 'parents' of eukaryotes reveal complicated evolutionary relationships. Although many of the bacterial-like genes in eukaryotes take α-proteobacterial homologues, these are far from ascendant amongst the bacterial-like genes which show apparent evolutionary affinities with a variety of bacterial groups (Effigy 2). An important cause of this complicated breakdown of the bacterial-like component of the eukaryotic gene complement is the large size of the α-proteobacterial pangenome, that is, of the combined genes found in all α-proteobacteria, and the associated diversity of the cistron sets in individual members of this group [82]. Thus, without knowing the exact identity within the α-proteobacteria of the bacterial endosymbiont that gave rise to the eukaryotic mitochondria, it is hard to delineate its genetic contribution. Apart from this doubtfulness near the factor complement of the endosymbiont, it is incommunicable to rule out multiple sources of the bacterial-similar genes in eukaryotes [83], which may have origins other than the genome of the bacterial endosymbiont. In item, whatever the actual nature of the archaeal-similar ancestor, it probably lived at moderate temperatures and non-extreme weather condition and was consequently in contact with a various bacterial community. Modern archaea with such lifestyles have numerous genes of diverse bacterial origins, indicating extensive horizontal conquering of genes from leaner [84, 85]. Thus, the archaeal-similar host of the endosymbiont could have already had many bacterial genes, partly explaining the observed pattern.
The case of the archaeal(-like) parent is far more difficult than that of the bacterial ancestor(southward) as there are no data on the ancestral lineage that would parallel the unambiguous origin of mitochondria from α-proteobacteria. Phylogenomic studies using different methods point to different archaeal lineages - Crenarchaeota [86, 87], Euryarchaeota [88], or an unidentified deep branch [89, 90] - as the candidates for the eukaryote ancestor (Figure 3). Unequivocal resolution of such deep evolutionary relationships is extremely difficult. Moreover, at least i of these analyses [89] explicitly suggests the possibility that the archaeal heritage of eukaryotes is genuinely mixed, with the largest contribution coming from a deep lineage, followed by the contributions from Crenarchaeota (Thaumoarchaeota) and the Euryarchaeota (Figure 3). In the next department I examine the possibility of multiple archaeal and bacterial ancestors of the eukaryotes with respect to singled-out functional systems of eukaryotic cells.
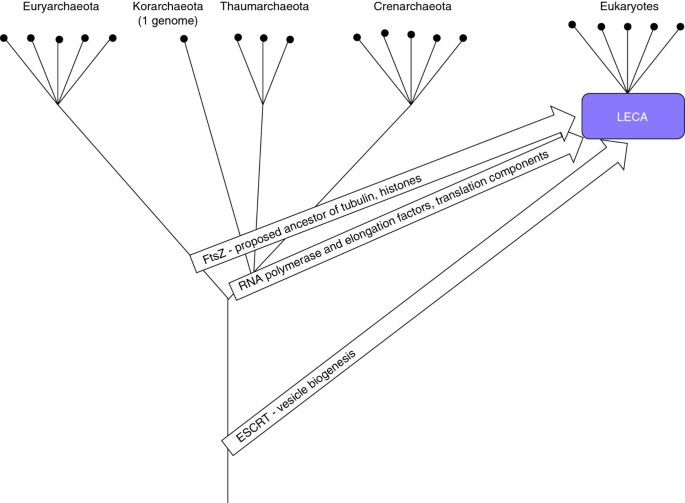
Possible archaeal origins of eukaryotic genes. The archaeal tree is shown every bit a bifurcation of Euryarchaeota and the putative second major branch combining Crenarchaeota, Thaumarchaeota, and Korarchaeota [127]; deep, possibly extinct lineages are shown as a single stem.
Mixed origins of the key functional systems of eukaryotes
Some of the most compelling indications on the course of evolution and the nature of bequeathed forms come from signature genes that are uniquely shared by 2 or more major lineages and from detailed evolutionary analysis of well characterized functional systems, in item the signature systems of the eukaryotic cell. Comparative genome sequence analysis has revealed that some of the key molecular machines of the eukaryotes, and not only those directly involved in information processing, can be confidently derived from archaeal ancestors (Table 1 and Figure iv). Strikingly, this archaeal heritage seems to exist patchy with respect to the specific origins, with apparent evolutionary affinities to unlike groups of archaea (Table one and Figure iv). For instance, comparative analysis of the translation system components tends to suggest an analogousness between eukaryotes and Crenarchaeota [91]. Similarly, the core transcription machinery of eukaryotes shares some of import proteins with Crenarchaeota, Thaumarchaeota and Korarchaeota, to the exclusion of Euryarchaeota [92–94]. By contrast, the histones, the primary components of nucleosomes, are missing in almost of the Crenarchaeota but invariably conserved in Euryarchaeota (and likewise present in Korarchaeum and some Thaumarchaeota) [95].
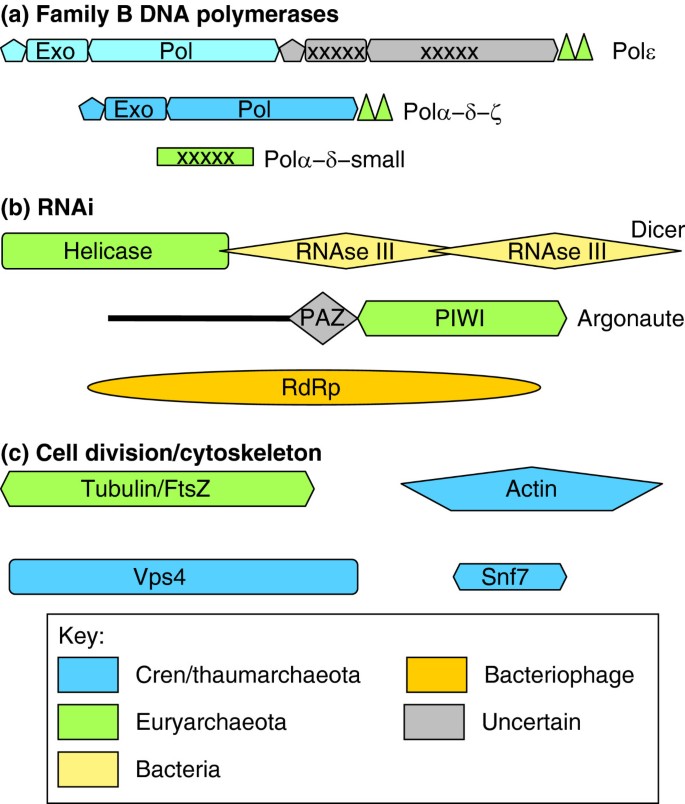
Credible circuitous origins of some key functional systems of eukaryotes. The likely origins of proteins and domains are shown by color lawmaking for three central functional systems of the eukaryotic jail cell: (a) B-family Dna polymerases comprising the core of the replication apparatus (triangles prove Zn-finger modules; crosses bespeak inactivated enzymatic domains; pol, polymerase; exo, exonuclease) [100]; (b) RNA interference (RNAi) machinery (RdRp, RNA-dependent RNA polymerase) [70]; and (c) cell division appliance (the Vps4 ATPase and Snf7-like proteins comprise the ESCRT-III machinery) and cytoskeleton [97, 98, 105, 113]. The domains are non drawn to scale. The calorie-free bluish color of the iii amino-terminal domains of Polε indicates the substantial sequence divergence from the homologous domains of other eukaryotic polymerases.
Eukaryotic cell division components are also conserved in several simply not all of the major archaeal lineages. For example, homologs of the ESCRT-III complex, which performs key roles in vesicle biogenesis and cytokinesis in eukaryotes, are responsible for cell segmentation in the Crenarchaeota but are missing in most of the Euryarchaeota, which possess a bacterial-like sectionalization machinery using the GTPase FtsZ, a distant homolog of tubulin [96, 97]. However, a few members of the Euryarchaeota accept both systems, with FtsZ probably responsible for partition and ESCRT-Iii for vesicle biogenesis [98].
Eukaryote B-family DNA polymerases, a group of four paralogs that are collectively responsible for genome replication, evidence a circuitous design of beginnings (Effigy four): one branch of the eukaryotic polymerases seems to have evolved from archaeal PolBI, which is conserved in all archaea, whereas the other branch appears to derive from the Crenarchaea-specific PolBII [99, 100]. Surprisingly, the eukaryotic polymerases additionally comprise a Zn-finger domain homologous to that of PolD, which is restricted to Euryarchaeota [100]; furthermore, the small subunits of eukaryotic Polα and Polδ are inactivated derivatives of the exonuclease subunit of PolD [101].
Some other major theme emerging from these studies is the bacterial contribution and the formation of archaeao-bacterial chimeras (Table 1 and Figure iv). A clear-cut case of a chimeric eukaryotic system is the RNA interference mechanism, in which one of the central proteins, the endonuclease Dicer, consists of two bacterial RNAse Iii domains and a helicase domain of apparent euryarchaeal origin, and the other essential poly peptide, Argonaute, as well shows a euryarchaeal affinity (Figure 4) [70, 102]. The nuclear pore circuitous, a quintessential eukaryotic molecular auto, does not show whatever indications of archaeal ancestry but rather consists of proteins of credible bacterial origin combined with proteins consisting of elementary repeats whose provenance is hard to ascertain [28].
These observations suggest that the archaeal antecedent of eukaryotes combined a variety of features constitute separately in various extant archaea. This inference is consequent with the results of phylogenomic analysis and evolutionary reconstruction discussed in a higher place. Thus, the currently existing archaeal lineages probably evolved by differential streamlining, or reductive evolution of the complex ancestral forms, whereas eukaryotes largely retained the ancestral complexity. The diverse origins of eukaryotic functional systems has major implications for how eukaryotes originated, equally explained below.
Eukaryogenesis: where did the eukaryotes come from?
The results of comparative genomics and ultrastructural studies do non however definitively evidence where the eukaryotic cell came from, but they do offer important insights. Box 1 lists the cardinal observations that must be included in any evolutionary scenario for the evolution of eukaryotes (called eukaryogenesis) and summarizes the 2 alternative scenarios, which are depicted in Figure v. The main result revolves effectually the office of endosymbiosis [ii, 3, 103, 104]: was it the cause of the entire chain of events that led to the emergence of LECA (the stalk stage of evolution), as proposed by the symbiogenesis scenario, or was information technology a step in the evolution of the already formed eukaryotic cell, every bit proposed by the archaezoan scenario? In other words, was the host of the α-proteobacterial symbiont (the futurity mitochondrion) a prokaryote (as in the symbiogenesis scenario) or an amitochondrial eukaryote, an archaezoan?
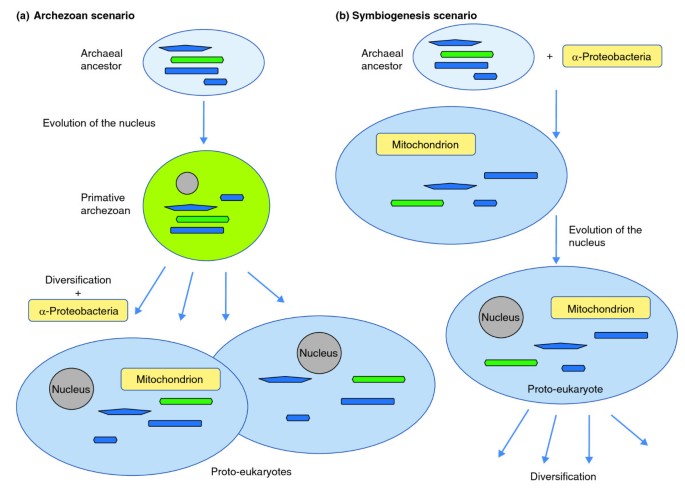
The two culling scenarios of eukaryogenesis. (a) The archaezoan scenario; (b) the symbiogenesis scenario. The putative archaeal or archaezoan hosts of the α-proteobacterial endosymbiont are shown with elements of their cytoskeleton and cell partition appliance colored as in Figure 4.
General concepts in the evolution of the eukaryotes
Given that eukaryogenesis may accept been a unique event and that intermediate stages in the process cannot be seen, these questions are enormously difficult, and final answers might not be attainable. But the symbiogenesis scenario seems to be more plausible than the archaezoan scenario [105], for three master reasons. Kickoff, under the archaezoan scenario, there is no plausible selective force backside the development of the nucleus, and in particular the elaborate nuclear pore circuitous. The nucleus disrupts the transcription-translation coupling that is typical of bacteria and archaea [106–108] and necessitates the evolution of the fourth dimension- and free energy-consuming machinery of nucleocytosolic transport of mRNA. By contrast, the symbiogenesis hypothesis offers a plausible selective cistron: defence against the invasion of the host genome by Group II cocky-splicing introns, which are abundant in α-proteobacteria and could have been unleashed as a effect of exposure of the archaeal host genome to the bacterial endosymbiont DNA; these would disrupt gene expression unless transcription and translation were decoupled and compartmentalized [106]. At least some boosted innovations of eukaryogenesis, such as the evolution of the nonsense-mediated decay of transcripts containing premature stop codons and expansion of the ubiquitin organization, can be envisaged every bit part of the same concatenation of adaptations to the intron bombardment as the origin of the nucleus [109] (Figure five).
Second, functional studies in prokaryotes, especially archaea, show that non only the molecular components of the several signature eukaryotic systems simply also their actual structures and functions have evolved in archaea and thus predate eukaryogenesis. These include the archaeal proteasome [110], exosome [111] and Sm-protein complex, the progenitor of the spliceosome [112], the ESCRT-Three membrane remodeling system [113, 114], actin-similar proteins [105] and a epitome of the ubiquitin system of protein modification [115]. Each of these molecular machines found in different groups of archaea has been shown or predicted to be mechanistically similar to the eukaryotic counterpart, but they all function within the prokaryotic cell. The endomembrane organization and the nucleus are dramatic exceptions, and so are the mitochondria themselves. It is tempting to connect these dots past proposing that eukaryogenesis was triggered by endosymbiosis, and that the endomembrane systems including the nucleus evolved as defense against invasion of Group Ii introns and perhaps strange Deoxyribonucleic acid in general [106, 109]. It does non seem accidental that many cardinal components of these endomembrane systems seem to exist of bacterial origin whereas others are repetitive proteins that might have evolved de novo [28]. Under the symbiogenesis scenario, various pre-existing systems of the archaeal host were co-opted and expanded within the emerging eukaryotic cellular arrangement [66].
Several arguments tin exist and have been put forward against the symbiogenesis scenario. First, prokaryotic endosymbionts in prokaryotic hosts are not widespread, prompting the view that phagocytosis, which is plainly unique to eukaryotic cells, was critical for the acquisition of the mitochondrion [3]. This argument is not compelling because: (i) eukaryogenesis is extremely rare, probably unique, in the history of life; (2) endosymbiotic bacteria within other bacteria are rare but known [116–118], and intracellular bacterial predation has been suggested as a potential road to endosymbiosis [119]; and (3) contempo observations on membrane remodeling systems and actin-like proteins in archaea suggest the possibility of still unexplored mechanisms for engulfment of other prokaryotes, perhaps resembling primitive phagocytosis [105].
2d, a potentially strong argument against the symbiogenesis scenario could be the existence of a substantial number of eukaryote signature proteins (ESPs), so far institute just in eukaryotes [120]. The provenance of ESPs is an intriguing question. Yet, on many occasions, careful sequence and construction searches have revealed archaeal and/or bacterial homologs of proteins originally considered ESPs, or else the existence of such homologs became obvious with the appearance of new genomes [66]. The discovery of prokaryotic homologs of tubulin, actin and ubiquitin are well known examples [71, 97], and more recent cases include the GINS proteins, which are involved in DNA replication [121], the ESCRT-III systems and the subunits of the TRAPP3 complex, which take a cardinal function in eukaryotic vesicle trafficking [122]. Nether the symbiogenesis scenario, the one-time and remaining ESPs result primarily from acceleration of evolution of genes whose functions have essentially changed during eukaryogenesis.
A third, potentially serious difficulty with the symbiogenesis scenario is that neither archaeal-like nor bacterial-like genes can exist traced to a unmarried prokaryotic lineage (although the origin of the mitochondria from α-proteobacteria is well established). However, the pangenomes of prokaryotes are large whereas the gene composition of individual organisms is highly flexible [123, 124], and so reconstruction of the bodily partners of the endosymbiosis that led to eukaryogenesis might not be feasible from a express set of extant genomes. Moreover, many if not most archaea and bacteria might take evolved by streamlining, and so eukaryogenesis could accept been triggered by symbiosis between 2 prokaryotes with complex genomes.
In short, it is currently incommunicable to strictly rule out the possibility that the key eukaryotic innovations evolved independently from and prior to the mitochondrial endosymbiosis. In other words, the host of the endosymbiont might accept been an archaezoan. However, the archaezoan scenario does not provide a plausible staging of events during the evolution of the complex internal organisation of the eukaryotic cell, does not offer a raison d'être for the nucleus, and does not account for the presence of signature functional systems of eukaryotes in different archaeal lineages. In dissimilarity, the symbiogenesis scenario tin can tie all these diverse lines of evidence into a coherent, even if even so woefully incomplete, narrative.
Comparative genomics has so far neither solved the enigma of eukaryogenesis nor offered a definitive picture of the principal radiation of the major eukaryote lineages. Notwithstanding, although falling short of decisive answers, phylogenomic analysis has yielded many insights into the origin and primeval stages of evolution of eukaryotes. Contempo findings indicate that several cardinal cellular systems of eukaryotes be in archaea. The scattering of these systems among different archaeal lineages, forth with the phylogenies of conserved proteins, suggests that the archaeal ancestor of eukaryotes belonged to a deep, possibly extinct archaeal branch with a highly complex genome and various cellular functionalities. In contrast, the endomembrane systems of eukaryotes, and in item the nucleus with its elaborate nuclear pore complex, are not found in archaea, and seem to be derived, at least in part, from bacterial ancestral components. These findings seem to be best uniform with a symbiogenesis scenario for the origin of eukaryotes under which eukaryogenesis was triggered by the endosymbiosis of an α-proteobacterium with an ancestral archaeon, with the nucleus evolving as a defense confronting intron invasion.
Phylogenomic analysis has antiseptic the evolutionary links betwixt major groups of eukaryotes and led to the depiction of five or 6 supergroups. The relationships betwixt the supergroups and the root position in the tree of eukaryotes remain extremely hard to decipher, probably owing to a compressed cladogenesis or 'big bang' phase of evolution that followed eukaryogenesis. The expanding sampling of genomes from various branches of life is far from being a trivial pursuit, merely has rather delivered unexpected biological insights.
References
-
Dacks JB, Doolittle WF: Reconstructing/deconstructing the earliest eukaryotes: how comparative genomics tin assistance. Cell. 2001, 107: 419-425. 10.1016/S0092-8674(01)00584-0.
-
Embley TM, Martin Due west: Eukaryotic evolution, changes and challenges. Nature. 2006, 440: 623-630. 10.1038/nature04546.
-
Kurland CG, Collins LJ, Penny D: Genomics and the irreducible nature of eukaryote cells. Science. 2006, 312: 1011-1014. ten.1126/science.1121674.
-
Ovadi J, Saks V: On the origin of intracellular compartmentation and organized metabolic systems. Mol Cell Biochem. 2004, 256-257: 5-12. ten.1023/B:MCBI.0000009855.14648.2c.
-
Martin W: Evolutionary origins of metabolic compartmentalization in eukaryotes. Philos Trans R Soc Lond B Biol Sci. 365: 847-855.
-
Jekely G: Origin of eukaryotic endomembranes: a critical evaluation of different model scenarios. Adv Exp Med Biol. 2007, 607: 38-51. full_text.
-
Hudder A, Nathanson L, Deutscher MP: Organization of mammalian cytoplasm. Mol Cell Biol. 2003, 23: 9318-9326. 10.1128/MCB.23.24.9318-9326.2003.
-
Guigas Yard, Kalla C, Weiss One thousand: The degree of macromolecular crowding in the cytoplasm and nucleoplasm of mammalian cells is conserved. FEBS Lett. 2007, 581: 5094-5098. ten.1016/j.febslet.2007.09.054.
-
Dacks JB, Peden AA, Field MC: Evolution of specificity in the eukaryotic endomembrane organisation. Int J Biochem Jail cell Biol. 2009, 41: 330-340. x.1016/j.biocel.2008.08.041.
-
Field MC, Dacks JB: Starting time and concluding ancestors: reconstructing evolution of the endomembrane system with ESCRTs, vesicle coat proteins, and nuclear pore complexes. Curr Opin Jail cell Biol. 2009, 21: 4-13. x.1016/j.ceb.2008.12.004.
-
Giezen van der M, Tovar J: Degenerate mitochondria. EMBO Rep. 2005, 6: 525-530. 10.1038/sj.embor.7400440.
-
Hjort Chiliad, Goldberg AV, Tsaousis Ad, Hirt RP, Embley TM: Diversity and reductive evolution of mitochondria among microbial eukaryotes. Philos Trans R Soc Lond B Biol Sci. 365: 713-727.
-
Giezen van der M: Hydrogenosomes and mitosomes: conservation and evolution of functions. J Eukaryot Microbiol. 2009, 56: 221-231. 10.1111/j.1550-7408.2009.00407.x.
-
Hunter T: The age of crosstalk: phosphorylation, ubiquitination, and across. Mol Prison cell. 2007, 28: 730-738. 10.1016/j.molcel.2007.11.019.
-
Hubbard SR, Till JH: Protein tyrosine kinase structure and function. Annu Rev Biochem. 2000, 69: 373-398. x.1146/annurev.biochem.69.ane.373.
-
Pawson T, Kofler M: Kinome signaling through regulated poly peptide-poly peptide interactions in normal and cancer cells. Curr Opin Cell Biol. 2009, 21: 147-153. 10.1016/j.ceb.2009.02.005.
-
Pawson T: Specificity in signal transduction: from phosphotyrosine-SH2 domain interactions to complex cellular systems. Jail cell. 2004, 116: 191-203. 10.1016/S0092-8674(03)01077-viii.
-
Johnson SA, Hunter T: Kinomics: methods for deciphering the kinome. Nat Methods. 2005, 2: 17-25. 10.1038/nmeth731.
-
Dikic I, Wakatsuki S, Walters KJ: Ubiquitin-binding domains - from structures to functions. Nat Rev Mol Jail cell Biol. 2009, ten: 659-671. x.1038/nrm2767.
-
Hershko A, Ciechanover A: The ubiquitin system. Annu Rev Biochem. 1998, 67: 425-479. x.1146/annurev.biochem.67.ane.425.
-
Ciechanover A, Orian A, Schwartz AL: Ubiquitin-mediated proteolysis: biological regulation via destruction. Bioessays. 2000, 22: 442-451. 10.1002/(SICI)1521-1878(200005)22:5<442::AID-BIES6>3.0.CO;two-Q.
-
Liu Q, Paroo Z: Biochemical principles of small RNA pathways. Annu Rev Biochem. 2010,
-
Chapman EJ, Carrington JC: Specialization and evolution of endogenous small RNA pathways. Nat Rev Genet. 2007, 8: 884-896. 10.1038/nrg2179.
-
Amaral PP, Dinger ME, Mercer TR, Mattick JS: The eukaryotic genome as an RNA machine. Science. 2008, 319: 1787-1789. 10.1126/science.1155472.
-
Jacquier A: The circuitous eukaryotic transcriptome: unexpected pervasive transcription and novel pocket-sized RNAs. Nat Rev Genet. 2009, x: 833-844. x.1038/nrg2683.
-
D'Alessio JA, Wright KJ, Tjian R: Shifting players and paradigms in cell-specific transcription. Mol Jail cell. 2009, 36: 924-931. 10.1016/j.molcel.2009.12.011.
-
Wilson Physician, Odom DT: Development of transcriptional control in mammals. Curr Opin Genet Dev. 2009, 19: 579-585. ten.1016/j.gde.2009.x.003.
-
Mans BJ, Anantharaman 5, Aravind L, Koonin EV: Comparative genomics, development and origins of the nuclear envelope and nuclear pore complex. Cell Cycle. 2004, 3: 1612-1637.
-
Collins 50, Penny D: Complex spliceosomal organization ancestral to extant eukaryotes. Mol Biol Evol. 2005, 22: 1053-1066. 10.1093/molbev/msi091.
-
Dagan T, Artzy-Randrup Y, Martin W: Modular networks and cumulative impact of lateral transfer in prokaryote genome development. Proc Natl Acad Sci Usa. 2008, 105: 10039-10044. 10.1073/pnas.0800679105.
-
Entrez Genome. [http://www.ncbi.nlm.nih.gov/sites/genome]
-
Roger AJ: Reconstructing early events in eukaryotic development. Am Nat. 1999, 154 Suppl iv: S146-S163. 10.1086/303290.
-
Sogin ML, Morrison HG, Hinkle G, Silberman JD: Ancestral relationships of the major eukaryotic lineages. Microbiologia. 1996, 12: 17-28.
-
Baldauf SL, Roger AJ, Wenk-Siefert I, Doolittle WF: A kingdom-level phylogeny of eukaryotes based on combined protein information. Scientific discipline. 2000, 290: 972-977. ten.1126/science.290.5493.972.
-
Baldauf SL: The deep roots of eukaryotes. Science. 2003, 300: 1703-1706. x.1126/science.1085544.
-
Roger AJ, Hug LA: The origin and diversification of eukaryotes: issues with molecular phylogenetics and molecular clock estimation. Philos Trans R Soc Lond B Biol Sci. 2006, 361: 1039-1054. 10.1098/rstb.2006.1845.
-
Brinkmann H, Philippe H: The multifariousness of eukaryotes and the root of the eukaryotic tree. Adv Exp Med Biol. 2007, 607: 20-37. full_text.
-
Keeling PJ, McFadden GI: Origins of microsporidia. Trends Microbiol. 1998, half dozen: 19-23. x.1016/S0966-842X(97)01185-two.
-
Philippe H, Germot A, Moreira D: The new phylogeny of eukaryotes. Curr Opin Genet Dev. 2000, 10: 596-601. 10.1016/S0959-437X(00)00137-4.
-
Minge MA, Silberman JD, Orr RJ, Condescending-Smith T, Shalchian-Tabrizi K, Burki F, Skjaeveland A, Jakobsen KS: Evolutionary position of breviate amoebae and the master eukaryote divergence. Proc Biol Sci. 2009, 276: 597-604. 10.1098/rspb.2008.1358.
-
Boussau B, Daubin V: Genomes as documents of evolutionary history. Trends Ecol Evol. 2009, 25: 224-232. ten.1016/j.tree.2009.09.007.
-
Delsuc F, Brinkmann H, Philippe H: Phylogenomics and the reconstruction of the tree of life. Nat Rev Genet. 2005, vi: 361-375. ten.1038/nrg1603.
-
Adl SM, Simpson AG, Farmer MA, Andersen RA, Anderson OR, Barta JR, Bowser SS, Brugerolle Yard, Fensome RA, Fredericq S, James TY, Karpov S, Kugrens P, Krug J, Lane CE, Lewis LA, Lodge J, Lynn DH, Mann DG, McCourt RM, Mendoza L, Moestrup O, Mozley-Standridge SE, Nerad TA, Shearer CA, Smirnov AV, Spiegel FW, Taylor MF: The new college level classification of eukaryotes with emphasis on the taxonomy of protists. J Eukaryot Microbiol. 2005, 52: 399-451. 10.1111/j.1550-7408.2005.00053.x.
-
Keeling PJ: Genomics. Deep questions in the tree of life. Science. 2007, 317: 1875-1876. ten.1126/science.1149593.
-
Keeling PJ, Burger 1000, Durnford DG, Lang BF, Lee RW, Pearlman RE, Roger AJ, Gray MW: The tree of eukaryotes. Trends Ecol Evol. 2005, twenty: 670-676. 10.1016/j.tree.2005.09.005.
-
Parfrey LW, Barbero Due east, Lasser E, Dunthorn M, Bhattacharya D, Patterson DJ, Katz LA: Evaluating support for the current nomenclature of eukaryotic multifariousness. PLoS Genet. 2006, 2: e220-10.1371/periodical.pgen.0020220.
-
Burki F, Shalchian-Tabrizi Thou, Minge Grand, Skjaeveland A, Nikolaev SI, Jakobsen KS, Pawlowski J: Phylogenomics reshuffles the eukaryotic supergroups. PLoS ONE. 2007, 2: e790-x.1371/journal.pone.0000790.
-
Yoon HS, Grant J, Tekle YI, Wu Thou, Chaon BC, Cole JC, Logsdon JM, Patterson DJ, Bhattacharya D, Katz LA: Broadly sampled multigene trees of eukaryotes. BMC Evol Biol. 2008, 8: fourteen-10.1186/1471-2148-eight-14.
-
Moreira D, Heyden von der South, Bass D, Lopez-Garcia P, Chao E, Cavalier-Smith T: Global eukaryote phylogeny: combined small- and large-subunit ribosomal Deoxyribonucleic acid trees support monophyly of Rhizaria, Retaria and Excavata. Mol Phylogenet Evol. 2007, 44: 255-266. x.1016/j.ympev.2006.11.001.
-
Hackett JD, Yoon HS, Li S, Reyes-Prieto A, Rummele SE, Bhattacharya D: Phylogenomic assay supports the monophyly of cryptophytes and haptophytes and the clan of rhizaria with chromalveolates. Mol Biol Evol. 2007, 24: 1702-1713. 10.1093/molbev/msm089.
-
Hampl Five, Hug L, Leigh JW, Dacks JB, Lang BF, Simpson AG, Roger AJ: Phylogenomic analyses support the monophyly of Excavata and resolve relationships amidst eukaryotic "supergroups". Proc Natl Acad Sci USA. 2009, 106: 3859-3864. 10.1073/pnas.0807880106.
-
Burki F, Shalchian-Tabrizi G, Pawlowski J: Phylogenomics reveals a new 'megagroup' including well-nigh photosynthetic eukaryotes. Biol Lett. 2008, 4: 366-369. x.1098/rsbl.2008.0224.
-
Germot A, Philippe H: Disquisitional analysis of eukaryotic phylogeny: a case study based on the HSP70 family. J Eukaryot Microbiol. 1999, 46: 116-124. 10.1111/j.1550-7408.1999.tb04594.ten.
-
Rokas A, Kruger D, Carroll SB: Fauna evolution and the molecular signature of radiations compressed in time. Scientific discipline. 2005, 310: 1933-1938. 10.1126/science.1116759.
-
Koonin EV: The Biological Big Bang model for the major transitions in development. Biol Direct. 2007, 2: 21-10.1186/1745-6150-2-21.
-
Stechmann A, Cavalier-Smith T: Rooting the eukaryote tree by using a derived gene fusion. Scientific discipline. 2002, 297: 89-91. 10.1126/scientific discipline.1071196.
-
Stechmann A, Cavalier-Smith T: The root of the eukaryote tree pinpointed. Curr Biol. 2003, 13: R665-R666. 10.1016/S0960-9822(03)00602-X.
-
Richards TA, Cavalier-Smith T: Myosin domain development and the master deviation of eukaryotes. Nature. 2005, 436: 1113-1118. 10.1038/nature03949.
-
Rokas A, Holland Prisoner of war: Rare genomic changes as a tool for phylogenetics. Trends Ecol Evol. 2000, 15: 454-459. x.1016/S0169-5347(00)01967-four.
-
Rogozin IB, Basu MK, Csuros Chiliad, Koonin EV: Analysis of rare genomic changes does not support the unikont-bikont phylogeny and suggests cyanobacterial symbiosis equally the signal of primary radiation of eukaryotes. Genome Biol Evol. 2009, 2009: 99-113.
-
Rokas A, Carroll SB: Bushes in the tree of life. PLoS Biol. 2006, 4: e352-10.1371/journal.pbio.0040352.
-
Koonin EV, Fedorova ND, Jackson JD, Jacobs AR, Krylov DM, Makarova KS, Mazumder R, Mekhedov SL, Nikolskaya AN, Rao BS, Rogozin IB, Smirnov S, Sorokin AV, Sverdlov AV, Vasudevan Due south, Wolf YI, Yin JJ, Natale DA: A comprehensive evolutionary classification of proteins encoded in consummate eukaryotic genomes. Genome Biol. 2004, 5: R7-10.1186/gb-2004-5-two-r7.
-
Makarova KS, Wolf YI, Mekhedov SL, Mirkin BG, Koonin EV: Ancestral paralogs and pseudoparalogs and their part in the emergence of the eukaryotic cell. Nucleic Acids Res. 2005, 33: 4626-4638. 10.1093/nar/gki775.
-
Csuros M, Miklos I: Streamlining and large ancestral genomes in Archaea inferred with a phylogenetic nascency-and-decease model. Mol Biol Evol. 2009, 26: 2087-2095. 10.1093/molbev/msp123.
-
Ceulemans H, Beke L, Bollen Grand: Approaches to defining the ancestral eukaryotic protein complexome. Bioessays. 2006, 28: 316-324. ten.1002/bies.20373.
-
Aravind L, Iyer LM, Koonin EV: Comparative genomics and structural biology of the molecular innovations of eukaryotes. Curr Opin Struct Biol. 2006, 16: 409-419. 10.1016/j.sbi.2006.04.006.
-
Fritz-Laylin LK, Prochnik SE, Ginger ML, Dacks JB, Carpenter ML, Field MC, Kuo A, Paredez A, Chapman J, Pham J, Shu S, Neupane R, Cipriano 1000, Mancuso J, Tu H, Salamov A, Lindquist E, Shapiro H, Lucas S, Grigoriev IV, Cande WZ, Fulton C, Rokhsar DS, Dawson SC: The Genome of Naegleria gruberi illuminates early eukaryotic versatility. Cell. 2010, 140: 631-642. 10.1016/j.cell.2010.01.032.
-
Koonin EV: The incredible expanding ancestor of eukaryotes. Cell. 140: 606-608. 10.1016/j.cell.2010.02.022.
-
Bapteste Eastward, Charlebois RL, MacLeod D, Brochier C: The two tempos of nuclear pore complex evolution: highly adapting proteins in an ancient frozen structure. Genome Biol. 2005, 6: R85-ten.1186/gb-2005-vi-ten-r85.
-
Shabalina SA, Koonin EV: Origins and development of eukaryotic RNA interference. Trends Ecol Evol. 2008, 23: 578-587. x.1016/j.tree.2008.06.005.
-
Hochstrasser M: Origin and function of ubiquitin-like proteins. Nature. 2009, 458: 422-429. 10.1038/nature07958.
-
Carmel 50, Wolf YI, Rogozin IB, Koonin EV: Three distinct modes of intron dynamics in the development of eukaryotes. Genome Res. 2007, 17: 1034-1044. 10.1101/gr.6438607.
-
Csuros M, Rogozin IB, Koonin EV: Extremely intron-rich genes in the alveolate ancestors inferred with a flexible maximum-likelihood approach. Mol Biol Evol. 2008, 25: 903-911. ten.1093/molbev/msn039.
-
Roy SW: Intron-rich ancestors. Trends Genet. 2006, 22: 468-471. 10.1016/j.tig.2006.07.002.
-
Roy SW, Gilbert W: The evolution of spliceosomal introns: patterns, puzzles and progress. Nat Rev Genet. 2006, 7: 211-221.
-
Archibald JM: The puzzle of plastid evolution. Curr Biol. 2009, 19: R81-R88. 10.1016/j.cub.2008.eleven.067.
-
Elias M, Archibald JM: Sizing up the genomic footprint of endosymbiosis. Bioessays. 2009, 31: 1273-1279. x.1002/bies.200900117.
-
Esser C, Ahmadinejad N, Wiegand C, Rotte C, Sebastiani F, Gelius-Dietrich G, Henze K, Kretschmann E, Richly E, Leister D, Bryant D, Steel MA, Lockhart PJ, Penny D, Martin W: A genome phylogeny for mitochondria among alpha-proteobacteria and a predominantly eubacterial ancestry of yeast nuclear genes. Mol Biol Evol. 2004, 21: 1643-1660. ten.1093/molbev/msh160.
-
Rivera MC, Lake JA: The ring of life provides evidence for a genome fusion origin of eukaryotes. Nature. 2004, 431: 152-155. ten.1038/nature02848.
-
Martin W, Embley TM: Evolutionary biology: early evolution comes full circle. Nature. 2004, 431: 134-137. 10.1038/431134a.
-
Greyness MW, Burger G, Lang BF: Mitochondrial evolution. Science. 1999, 283: 1476-1481. ten.1126/scientific discipline.283.5407.1476.
-
Esser C, Martin W, Dagan T: The origin of mitochondria in light of a fluid prokaryotic chromosome model. Biol Lett. 2007, iii: 180-184. 10.1098/rsbl.2006.0582.
-
Doolittle WF: You are what you swallow: a cistron transfer ratchet could account for bacterial genes in eukaryotic nuclear genomes. Trends Genet. 1998, 14: 307-311. 10.1016/S0168-9525(98)01494-2.
-
Galagan JE, Nusbaum C, Roy A, Endrizzi MG, Macdonald P, FitzHugh W, Calvo S, Engels R, Smirnov South, Atnoor D, Brown A, Allen Northward, Naylor J, Stange-Thomann Due north, DeArellano One thousand, Johnson R, Linton L, McEwan P, McKernan One thousand, Talamas J, Tirrell A, Ye W, Zimmer A, Hairdresser RD, Cann I, Graham DE, Grahame DA, Guss AM, Hedderich R, Ingram-Smith C, et al: The genome of One thousand. acetivorans reveals extensive metabolic and physiological multifariousness. Genome Res. 2002, 12: 532-542. x.1101/gr.223902.
-
Koonin EV: Horizontal factor transfer: the path to maturity. Mol Microbiol. 2003, 50: 725-727. ten.1046/j.1365-2958.2003.03808.x.
-
Cox CJ, Foster PG, Hirt RP, Harris SR, Embley TM: The archaebacterial origin of eukaryotes. Proc Natl Acad Sci Usa. 2008, 105: 20356-20361. x.1073/pnas.0810647105.
-
Foster PG, Cox CJ, Embley TM: The primary divisions of life: a phylogenomic approach employing composition-heterogeneous methods. Philos Trans R Soc Lond B Biol Sci. 2009, 364: 2197-2207. 10.1098/rstb.2009.0034.
-
Pisani D, Cotton fiber JA, McInerney JO: Supertrees disentangle the chimerical origin of eukaryotic genomes. Mol Biol Evol. 2007, 24: 1752-1760. 10.1093/molbev/msm095.
-
Yutin N, Makarova KS, Mekhedov SL, Wolf YI, Koonin EV: The deep archaeal roots of eukaryotes. Mol Biol Evol. 2008, 25: 1619-1630. 10.1093/molbev/msn108.
-
Saruhashi S, Hamada K, Miyata D, Horiike T, Shinozawa T: Comprehensive analysis of the origin of eukaryotic genomes. Genes Genet Syst. 2008, 83: 285-291. 10.1266/ggs.83.285.
-
Vishwanath P, Favaretto P, Hartman H, Mohr SC, Smith TF: Ribosomal protein-sequence cake structure suggests complex prokaryotic evolution with implications for the origin of eukaryotes. Mol Phylogenet Evol. 2004, 33: 615-625. 10.1016/j.ympev.2004.07.003.
-
Korkhin Y, Unligil UM, Littlefield O, Nelson PJ, Stuart DI, Sigler PB, Bell SD, Abrescia NG: Evolution of circuitous RNA polymerases: the consummate archaeal RNA polymerase construction. PLoS Biol. 2009, 7: e102-ten.1371/periodical.pbio.1000102.
-
Blombach F, Makarova KS, Marrero J, Siebers B, Koonin EV, Oost van der J: Identification of an ortholog of the eukaryotic RNA polymerase III subunit RPC34 in Crenarchaeota and Thaumarchaeota suggests specialization of RNA polymerases for coding and non-coding RNAs in Archaea. Biol Direct. 2009, 4: 39-x.1186/1745-6150-4-39.
-
Daniels JP, Kelly S, Wickstead B, Gull K: Identification of a crenarchaeal orthologue of Elf1: implications for chromatin and transcription in Archaea. Biol Direct. 2009, 4: 24-ten.1186/1745-6150-iv-24.
-
Reeve JN, Bailey KA, Li WT, Marc F, Sandman Chiliad, Soares DJ: Archaeal histones: structures, stability and DNA binding. Biochem Soc Trans. 2004, 32: 227-230. 10.1042/BST0320227.
-
Vaughan Due south, Wickstead B, Dupe K, Addinall SG: Molecular development of FtsZ poly peptide sequences encoded within the genomes of archaea, bacteria, and eukaryota. J Mol Evol. 2004, 58: 19-29. 10.1007/s00239-003-2523-v.
-
Lowe J, Amos LA: Evolution of cytomotive filaments: the cytoskeleton from prokaryotes to eukaryotes. Int J Biochem Cell Biol. 2009, 41: 323-329. 10.1016/j.biocel.2008.08.010.
-
Lindas Air conditioning, Karlsson EA, Lindgren MT, Ettema TJ, Bernander R: A unique cell division machinery in the Archaea. Proc Natl Acad Sci USA. 2008, 105: 18942-18946. 10.1073/pnas.0809467105.
-
Filee J, Forterre P, Sen-Lin T, Laurent J: Evolution of DNA polymerase families: evidences for multiple cistron exchange between cellular and viral proteins. J Mol Evol. 2002, 54: 763-773. 10.1007/s00239-001-0078-x.
-
Tahirov Th, Makarova KS, Rogozin IB, Pavlov YI, Koonin EV: Evolution of Dna polymerases: an inactivated polymerase-exonuclease module in Pol epsilon and a chimeric origin of eukaryotic polymerases from two classes of archaeal ancestors. Biol Direct. 2009, 4: 11-10.1186/1745-6150-4-11.
-
Aravind L, Koonin EV: Phosphoesterase domains associated with DNA polymerases of diverse origins. Nucleic Acids Res. 1998, 26: 3746-3752. 10.1093/nar/26.16.3746.
-
Makarova KS, Wolf YI, Oost van der J, Koonin EV: Prokaryotic homologs of Argonaute proteins are predicted to part as key components of a novel arrangement of defense confronting mobile genetic elements. Biol Direct. 2009, 4: 29-10.1186/1745-6150-4-29.
-
Martin Due west, Dagan T, Koonin EV, Dipippo JL, Gogarten JP, Lake JA: The development of eukaryotes. Science. 2007, 316: 542-543. x.1126/science.316.5824.542c. author reply 542-543
-
Poole A, Penny D: Eukaryote development: engulfed past speculation. Nature. 2007, 447: 913-10.1038/447913a.
-
Yutin Due north, Wolf MY, Wolf YI, Koonin EV: The origins of phagocytosis and eukaryogenesis. Biol Direct. 2009, 4: 9-10.1186/1745-6150-4-nine.
-
Martin W, Koonin EV: Introns and the origin of nucleus-cytosol compartmentation. Nature. 2006, 440: 41-45. 10.1038/nature04531.
-
Martin W, Koonin EV: A positive definition of prokaryotes. Nature. 2006, 442: 868-10.1038/442868c.
-
French SL, Santangelo TJ, Beyer AL, Reeve JN: Transcription and translation are coupled in Archaea. Mol Biol Evol. 2007, 24: 893-895. 10.1093/molbev/msm007.
-
Koonin EV: The origin of introns and their office in eukaryogenesis: a compromise solution to the introns-early versus introns-tardily debate?. Biol Direct. 2006, 1: 22-x.1186/1745-6150-1-22.
-
Maupin-Furlow JA, Wilson HL, Kaczowka SJ, Ou MS: Proteasomes in the archaea: from structure to function. Front Biosci. 2000, five: D837-D865. x.2741/furlow.
-
Hartung S, Hopfner KP: Lessons from structural and biochemical studies on the archaeal exosome. Biochem Soc Trans. 2009, 37: 83-87. x.1042/BST0370083.
-
Mura C, Kozhukhovsky A, Gingery Thousand, Phillips M, Eisenberg D: The oligomerization and ligand-binding properties of Sm-similar archaeal proteins (SmAPs). Protein Sci. 2003, 12: 832-847. 10.1110/ps.0224703.
-
Samson RY, Bell SD: Ancient ESCRTs and the evolution of binary fission. Trends Microbiol. 2009, 17: 507-513. 10.1016/j.tim.2009.08.003.
-
Ettema TJ, Bernander R: Cell partition and the ESCRT circuitous: a surprise from the archaea. Commun Integr Biol. 2009, 2: 86-88.
-
Humbard MA, Miranda HV, Lim JM, Krause DJ, Pritz JR, Zhou Chiliad, Chen S, Wells L, Maupin-Furlow JA: Ubiquitin-like minor archaeal modifier proteins (SAMPs) in Haloferax volcanii . Nature. 463: 54-60. 10.1038/nature08659.
-
von Dohlen CD, Kohler South, Alsop ST, McManus WR: Mealybug beta-proteobacterial endosymbionts contain gamma-proteobacterial symbionts. Nature. 2001, 412: 433-436. ten.1038/35086563.
-
Thao ML, Gullan PJ, Baumann P: Secondary (gamma-Proteobacteria) endosymbionts infect the primary (beta-Proteobacteria) endosymbionts of mealybugs multiple times and coevolve with their hosts. Appl Environ Microbiol. 2002, 68: 3190-3197. 10.1128/AEM.68.seven.3190-3197.2002.
-
Hoffmeister Thousand, Martin W: Interspecific evolution: microbial symbiosis, endosymbiosis and gene transfer. Environ Microbiol. 2003, 5: 641-649. 10.1046/j.1462-2920.2003.00454.ten.
-
Davidov Y, Jurkevitch Due east: Predation betwixt prokaryotes and the origin of eukaryotes. Bioessays. 2009, 31: 748-757. 10.1002/bies.200900018.
-
Hartman H, Fedorov A: The origin of the eukaryotic prison cell: a genomic investigation. Proc Natl Acad Sci USA. 2002, 99: 1420-1425. 10.1073/pnas.032658599.
-
MacNeill SA: Structure and office of the GINS circuitous, a key component of the eukaryotic replisome. Biochem J. 425: 489-500. 10.1042/BJ20091531.
-
Podar M, Wall MA, Makarova KS, Koonin EV: The prokaryotic V4R domain is the probable ancestor of a key component of the eukaryotic vesicle send system. Biol Direct. 2008, iii: 2-10.1186/1745-6150-3-2.
-
Koonin EV, Wolf YI: Genomics of bacteria and archaea: the emerging dynamic view of the prokaryotic world. Nucleic Acids Res. 2008, 36: 6688-6719. x.1093/nar/gkn668.
-
Lapierre P, Gogarten JP: Estimating the size of the bacterial pan-genome. Trends Genet. 2009, 25: 107-110. 10.1016/j.tig.2008.12.004.
-
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ: Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997, 25: 3389-3402. 10.1093/nar/25.17.3389.
-
Koski LB, Golding GB: The closest Boom hitting is often not the nearest neighbor. J Mol Evol. 2001, 52: 540-542.
-
Elkins JG, Podar Yard, Graham DE, Makarova KS, Wolf Y, Randau L, Hedlund BP, Brochier-Armanet C, Kunin 5, Anderson I, Lapidus A, Goltsman E, Barry Yard, Koonin EV, Hugenholtz P, Kyrpides N, Wanner G, Richardson P, Keller Yard, Stetter KO: A korarchaeal genome reveals insights into the development of the Archaea. Proc Natl Acad Sci USA. 2008, 105: 8102-8107. 10.1073/pnas.0801980105.
-
Aravind L, Walker DR, Koonin EV: Conserved domains in DNA repair proteins and evolution of repair systems. Nucleic Acids Res. 1999, 27: 1223-1242. 10.1093/nar/27.five.1223.
-
Koonin EV, Makarova KS, Elkins JG: Orthologs of the small RPB8 subunit of the eukaryotic RNA polymerases are conserved in hyperthermophilic Crenarchaeota and "Korarchaeota". Biol Straight. 2007, 2: 38-ten.1186/1745-6150-2-38.
-
Anantharaman V, Koonin EV, Aravind Fifty: Comparative genomics and evolution of proteins involved in RNA metabolism. Nucleic Acids Res. 2002, 30: 1427-1464. x.1093/nar/30.7.1427.
-
Venancio TM, Balaji Due south, Iyer LM, Aravind 50: Reconstructing the ubiquitin network: cross-talk with other systems and identification of novel functions. Genome Biol. 2009, 10: R33-x.1186/gb-2009-10-3-r33.
-
Koonin EV, Wolf YI, Aravind 50: Prediction of the archaeal exosome and its connections with the proteasome and the translation and transcription machineries past a comparative-genomic arroyo. Genome Res. 2001, xi: 240-252. ten.1101/gr.162001.
-
Iyer LM, Anantharaman 5, Wolf MY, Aravind 50: Comparative genomics of transcription factors and chromatin proteins in parasitic protists and other eukaryotes. Int J Parasitol. 2008, 38: i-31. 10.1016/j.ijpara.2007.07.018.
-
Aravind 50, Iyer LM, Anantharaman 5: The 2 faces of Alba: the evolutionary connexion between proteins participating in chromatin construction and RNA metabolism. Genome Biol. 2003, iv: R64-ten.1186/gb-2003-four-10-r64.
-
Gray MW, Burger Thousand, Lang BF: The origin and early on evolution of mitochondria. Genome Biol. 2001, 2: reviews1018-10.1186/gb-2001-ii-6-reviews1018.
Acknowledgements
I give thanks Yuri Wolf for providing the data used in Figure 2, Nib Martin for helpful discussions and Tania Senkevich for critical reading of the manuscript. The author's research is supported by the DHHS (National Library of Medicine) intramural funds.
Author information
Affiliations
Corresponding author
Authors' original submitted files for images
Rights and permissions
About this article
Cite this commodity
Koonin, E.Five. The origin and early evolution of eukaryotes in the light of phylogenomics. Genome Biol eleven, 209 (2010). https://doi.org/10.1186/gb-2010-xi-5-209
-
Published:
-
DOI : https://doi.org/10.1186/gb-2010-11-5-209
Keywords
- Eukaryotic Cell
- Gene Complement
- Phylogenomic Analysis
- Endomembrane System
- Reductive Evolution
Source: https://genomebiology.biomedcentral.com/articles/10.1186/gb-2010-11-5-209
0 Response to "Evidence That Shows Agains the Origin of Eucaryotes"
Post a Comment